Citizen Writes
Research hot topics
Understanding and managing kidney disease
30/07/2021
By Izabella Pawluczyk, IgA nephropathy Research Group
Worldwide, one in ten people have chronic kidney disease, for which there is currently no cure. Treatments are largely based on controlling blood pressure and reducing loss of protein through the urine. Despite this, a significant number of affected people will end up on dialysis or need a kidney transplant.
Our group, led by Professor Jonathan Barratt, is a world leader in research into IgA nephropathy, (IgAN), a kidney disease that patients are usually diagnosed with in their late teens to 20s. It is a chronic condition that patients and their families live with for the rest of their lives.
IgA nephropathy is characterised by deposits of a protein called immunoglobulin A (IgA) in the kidneys’ filtering units. This causes inflammation, injury and scarring. Eventually the damage becomes so bad that the filter cannot work efficiently and eventually stops working altogether. At this point the patient will require dialysis or a kidney transplant. Approximately one third of patients who develop IgAN will eventually progress to this ‘end-stage’ of the disease. However, predicting which patients will do this has been a major challenge.
We are advancing new scientific knowledge, into the pathogenesis, diagnosis, prognosis and treatment of IgAN. One area of our research is in the field of microRNAs. These are very small pieces of ribonucleic acid (RNA) that fine-tune a multitude of important processes that go on in the body, such as development, cell proliferation, new blood vessel formation and programmed cell death. MicroRNAs differ from messenger RNA (mRNA) in that they are not directly involved in protein coding. A well-known example of coding mRNA is the mRNA in the Pfizer COVID-19 vaccine which carries the instructions to our cells to make the viral spike protein. This primes our immune system so that we can respond appropriately to the virus should we come into contact with it.
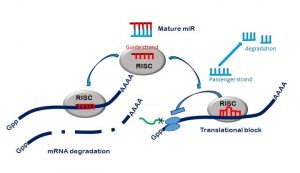 When a cell needs to make a new protein, for example one which limits growth and invasion of tumour cells, the blue print for making that protein is found in the DNA. The DNA helix unwinds to expose the gene coding for the protein. Messenger RNA (mRNA) copies this message and takes it to the protein making factories in the cell, called ribosomes. MicroRNAs will then fine tune this process ensuring the correct amount of protein is produced at any given time. MicroRNAs work by degrading mRNA so that the instructions for making a new protein cannot get to the ribosomes, or they can interfere directly with protein production. Irregular production of microRNAs can lead to the onset of disease.
When a cell needs to make a new protein, for example one which limits growth and invasion of tumour cells, the blue print for making that protein is found in the DNA. The DNA helix unwinds to expose the gene coding for the protein. Messenger RNA (mRNA) copies this message and takes it to the protein making factories in the cell, called ribosomes. MicroRNAs will then fine tune this process ensuring the correct amount of protein is produced at any given time. MicroRNAs work by degrading mRNA so that the instructions for making a new protein cannot get to the ribosomes, or they can interfere directly with protein production. Irregular production of microRNAs can lead to the onset of disease.
The presence of miRs in bodily fluids makes them very accessible and useful as potential biomarkers of disease. In addition they have also been the focus for use as targets for disease treatment.
The medical field is constantly on the lookout for ways of improving disease diagnosis, prognosis, and treatment. We have been trying to identify the microRNAs that can be used as biomarkers for predicting IgAN disease progression. Currently, the best tool for predicting this is the International IgAN risk prediction tool (IIgANRPT) – an algorithm that takes into account a number of patient demographics and clinical parameters such as age, gender, ethnicity and measure of kidney function. The algorithm then generates a risk score which can help doctors manage their patients and aids in the planning of their future clinical needs.
Our investigations have identified several microRNAs whose expression correlates with poor disease outcome [1,2]. The expression of one particular microRNA significantly improves the prognosis score of the IIgANRPT [3].
With unrivalled access to patient tissue samples, cells and biofluids from an extensive worldwide collaborative network we are able to validate new findings. We are currently collaborating with a group in China, where the disease is very common, to validate the addition of a miR to the IIgARPT.
For anyone wishing to get a more in depth view of the work that is being undertaken in the field of miRs in IgAN our comprehensive review is soon to be featured in a special issue of the Journal of Clinical Medicine [4].
- Izabella ZA Pawluczyk, Maria SF Soares, William A Barratt, Jeremy R Brown, Jasraj S Bhachu, Haresh Selvaskandan, Yiqing Zeng, Rishi Sarania, Karen Molyneux, Ian SD Roberts, Jonathan Barratt. Macrophage interactions with collecting duct epithelial cells are capable of driving tubulointerstitial inflammation and fibrosis in IgA Nephropathy. Nephrol Dial Transpl 35:1865-1877, 2020 doi 10.1093/ndt gfaa079
- Izabella ZA Pawluczyk, Athanasios Didangelos, Sean J Barbour, Lee Er, Jan U Becker, Roberto Martin, Scott Taylor, Jasraj S Bhachu, Edward G Lyons, Robert Jenkins, Donald Fraser, Karen Molyneux, Javier Perales-Patón, Julio Saez-Rodriguez, Jonathan Barratt. Differential expression of microRNAs in IgA Nephropathy: miR-150-5p, a potential mediator and marker of disease progression. Kidney Int, 99(5):1127-1139, 2021 doi 10.1016/j.kint 2020.12.028
- Izabella ZA Pawluczyk, Matthew Nicholson, Sean J Barbour, Lee Er, Haresh Selvaskandan, Jasraj Bhachu, Jonathan Barratt. A pilot study to predict risk of IgA nephropathy progression based on miR-204 expression. Kidney Int Reports 2021 (in press).
- IZA Pawluczyk, H Selvaskandan, J Barratt. The non-coding RNA landscape in IgA nephropathy – Where are we in 2021? Review: Journal of Clinical Medicine, 2021 (in press)